Abstract
Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19. Molnupiravir increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans. Here, we establish the molecular mechanisms underlying molnupiravir-induced RNA mutagenesis by the viral RNA-dependent RNA polymerase (RdRp). Biochemical assays show that the RdRp uses the active form of molnupiravir, β-d-N4-hydroxycytidine (NHC) triphosphate, as a substrate instead of cytidine triphosphate or uridine triphosphate. When the RdRp uses the resulting RNA as a template, NHC directs incorporation of either G or A, leading to mutated RNA products. Structural analysis of RdRp–RNA complexes that contain mutagenesis products shows that NHC can form stable base pairs with either G or A in the RdRp active center, explaining how the polymerase escapes proofreading and synthesizes mutated RNA. This two-step mutagenesis mechanism probably applies to various viral polymerases and can explain the broad-spectrum antiviral activity of molnupiravir.
Main
Coronaviruses use an RNA-dependent RNA polymerase (RdRp) for the replication and transcription of their RNA genome1,2,3,4,5,6. RdRp is an important target for the development of antiviral drugs against coronaviruses1,7,8,9,10. Structures of RdRp have been reported for SARS-CoV-111 and SARS-CoV-212,13,14,15,16 and provide insights into the mechanisms of RNA-dependent RNA synthesis17. The structures also enable mechanistic studies that can rationalize the molecular processes underlying the antiviral activity of compounds targeting RdRp.
Antiviral drugs often target viral polymerases and function as nucleoside analogs that terminate RNA chain elongation. However, such chain-terminating antivirals are generally not effective against SARS-CoV-2 because coronaviruses carry an exonucleolytic proofreading activity that can remove misincorporated nucleotides from the nascent RNA 3′ end18,19,20. The nucleoside analog remdesivir can circumvent proofreading because its incorporation does not terminate elongation but only stalls RdRp after the addition of three more nucleotides14,21,22,23,24. Remdesivir was the first FDA-approved drug for the treatment of patients with COVID-1925,26,27,28, but its effectiveness is disputed29, emphasizing the need to develop new antiviral drugs.
Another promising drug candidate for the treatment of patients with COVID-19 is molnupiravir (or EIDD-2801), which also targets the RdRp of SARS-CoV-2. Molnupiravir is an isopropylester prodrug of the nucleoside analog β-d-N4-hydroxycytidine (NHC or EIDD-1931)30,31. Molnupiravir interferes with the replication of various viruses30,31,32,33,34,35,36, including SARS-CoV-237,38. It inhibits SARS-CoV-2 replication in human lung tissue39, blocks SARS-CoV-2 transmission in ferrets40 and reduces SARS-CoV-2 RNA in patients41. In contrast to approved drugs such as remdesivir that are administered by infusion, molnupiravir is orally available. Molnupiravir has been tested in phase I trials42 for safety, tolerability and pharmacokinetics, and phase II/III studies are currently ongoing41 (NCT04405739, NCT04405570 and NCT04575597). Available data suggest that molnupiravir acts as a mutagenizing agent that causes an ‘error catastrophe’ during viral replication30,37,43. Indeed, NHC can introduce mutations into viral RNA, as shown for Venezuelan equine encephalitis virus44. Also, the sequencing of influenza virus populations has indicated that NHC causes G-to-A and C-to-U transitions in viral RNA30, and the same transitions have been found for SARS-CoV-237.
Despite this progress, a systematic biochemical and structural analysis of molnupiravir- or NHC-induced RNA mutagenesis by viral RNA polymerases is lacking. In this Article, we quantify the effects of molnupiravir or NHC on the RNA synthesis activity of SARS-CoV-2 RdRp using a purified biochemical system and defined synthetic RNAs. Together with structural analysis, we establish the molecular mechanism of molnupiravir-induced RNA mutagenesis. Our results provide detailed insights into the mechanism of action of molnupiravir, which is entirely distinct from that of remdesivir or chain-terminating nucleoside analogs.
Results
SARS-CoV-2 RdRp readily incorporates NHC into RNA
We first tested whether purified SARS-CoV-2 RdRp can use the active form of molnupiravir, NHC triphosphate (‘MTP’) (Fig. 1a,b), as a substrate for RNA synthesis. We conducted RNA elongation assays in a defined biochemical system using recombinant RdRp and synthetic RNA template–product duplexes (Methods). We used four different RNA duplexes that differed at position +1 of the template strand (Supplementary Table 1), which directs binding of the incoming nucleoside triphosphate (NTP) substrate (Fig. 1c). The RNA product strand contained a fluorescent label at its 5′ end that allowed us to monitor and quantify RNA elongation.
Fig. 1: RdRp incorporates NHC opposite G and A in the template.
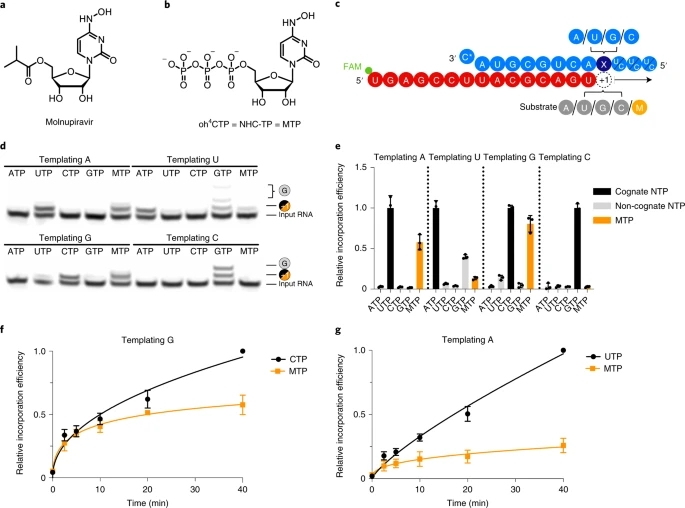
figure 1
a, Chemical structure of molnupiravir. b, Chemical structure of NHC triphosphate (MTP). c, The RNA template–product duplex. The direction of RNA extension is shown. The color of the depicted circles indicates the experimental design: blue, RNA template strand; dark blue, +1 templating nucleotide; red, RNA product strand; gray, NTP substrate; orange, MTP. The 5′ end of the RNA product contains a FAM fluorescent label. C* at the 3′ end of the template indicates dideoxy-C (ddC). d, NHC monophosphate is incorporated into growing RNA instead of C or U when G or A are present in the template +1 position. e, Quantification of nucleotide incorporation efficiency relative to the cognate NTP (dark gray) after triplicate measurements. Non-cognate NTPs and MTP are depicted in light gray and orange, respectively. Individual data points and boxes represent mean ± s.d. f, Quantification of time-dependent M incorporation opposite a templating G residue after triplicate measurements. Incorporation efficiency is calculated relative to cognate C incorporation. Data are mean ± s.d. g, Quantification of time-dependent M incorporation opposite a templating A residue after triplicate measurements. Incorporation efficiency is calculated relative to cognate U incorporation. Data are mean ± s.d. An uncropped gel image for d and data behind the graphs in e–g are available as source data.
Source data
Full size image
When nucleotides G or A were present at the RNA template position +1, NHC monophosphate (‘M’) was readily incorporated instead of C or U, respectively (Fig. 1d,e). Time-dependent RNA elongation experiments showed that M was slightly less efficiently incorporated than the cognate nucleotide C (Fig. 1f). M incorporation opposite A was also observed, but was substantially reduced compared to incorporation of the cognate nucleotide U (Fig. 1g). These results can be explained by base pairing of an incoming MTP substrate with either G or A in the RNA template strand. Consistent with this model, NHC adopts different tautomeric forms45 that have been predicted to allow for base pairing with either G or A46.
RdRp does not stall after NHC incorporation
We next tested whether the incorporation of NHC monophosphate (M) into nascent RNA interferes with further RNA extension. We first conducted RNA elongation assays with a scaffold that allowed for RNA extension by four nucleotides (nt) (Fig. 2a). We observed that incorporation of M instead of the cognate C or U did not prevent incorporation of three subsequent nucleotides (Fig. 2b). Furthermore, we tested RNA extension with a scaffold that allowed for the incorporation of 11 nt (Fig. 2c). In this case too, the RdRp reached the end of the template when uridine triphosphate (UTP) or cytidine triphosphate (CTP) was replaced by MTP, although, again, incorporation of M instead of U was less efficient than incorporation of M instead of C (Fig. 2d). These results demonstrate that M incorporation into nascent RNA does not prevent further RNA elongation. Thus, longer RNA products containing M nucleotides may be synthesized by the RdRp in the presence of MTP. This posed the question of what happens when M-containing RNA is used as a template in a second step of RNA synthesis.
Fig. 2: NHC incorporation does not stall SARS-CoV-2 RdRp.
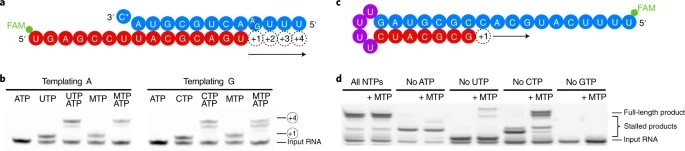
figure 2
a, The RNA template–product duplex (as in Fig. 1c) allows for RNA extension by four nucleotides. The direction of RNA extension is indicated. The 5′ end of the RNA product contains a FAM fluorescent label. C* at the 3′ end of the template indicates dideoxy-C (ddC). b, RNA elongation to the end of the template in a is possible when MTP replaces either CTP or UTP in the presence of adenosine triphosphate (ATP). The experiment was performed once. c, The RNA template–product hairpin duplex allows for RNA extension by 11 nucleotides. d, RNA elongation stalls at the expected positions when the cognate NTP is withheld from the reaction. Extension to the end of the template is possible when MTP replaces either CTP or UTP in the presence of other substrate NTPs, showing that incorporation of M does not prevent RNA extension. Note that more efficient RNA extension is seen at higher NTP/MTP concentrations, and also for MTP replacing UTP (not shown). The experiment was performed once. Uncropped gel images for b and d are available as source data.
Source data
Full size image
RdRp uses NHC-containing templates to direct RNA mutagenesis
To investigate the templating properties of NHC, we prepared an M-containing RNA by solid-phase synthesis using the phosphoramidite building block M-PA, which we synthesized using the convertible nucleoside approach from a ribose-protected O4-chlorophenyluridine (Fig. 3a, Methods, Extended Data Fig. 1 and Supplementary Dataset 1). The presence of M in the obtained RNA as well as RNA purity were confirmed by denaturing HPLC and HR-ESI-MS (Fig. 3b). The M-containing RNA oligo was annealed with a fluorescently labeled product RNA such that the M nucleotide occupied templating position +1 (Fig. 3c and Supplementary Table 1).
Fig. 3: NHC can direct incorporation of G and A into RNA.
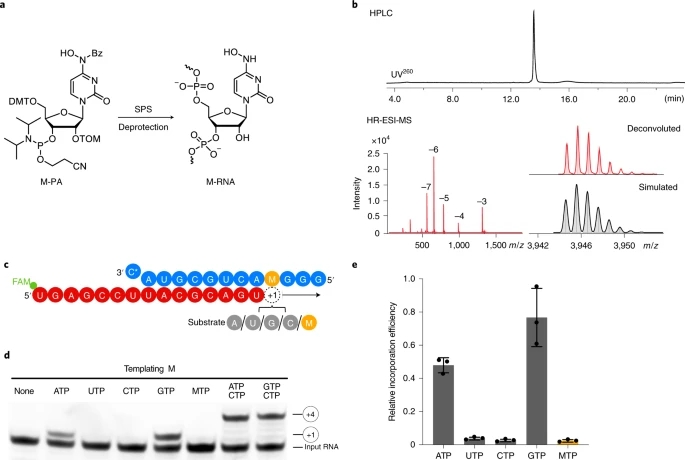
figure 3
a, Scheme of synthesis of RNA containing NHC monophosphate (M) at a defined position. 5′-O-DMT-2′-O-TOM-protected N4-hydroxycytidine phosphoramidite (M-PA) used for solid-phase synthesis of M-containing RNA (M-RNA). b, Analysis of M-containing RNA by denaturing HPLC confirms the homogeneity of the synthetic RNA (top). HR-ESI-MS analysis proves the presence of NHC and absence of unmodified RNA (bottom). c, The RNA template–product scaffold with M in template position +1, where it is used by the RdRp to direct binding of the incoming NTP substrate. The 5′ end of the RNA product contains a FAM fluorescent label. C* at the 3′ end of the template indicates dideoxy-C (ddC). d, When present at position +1 of the template strand, M can direct the incorporation of G or A into nascent RNA, but not C or U. e, Quantification of the experiment in d after triplicate measurements. Incorporation efficiencies are calculated relative to C incorporation opposite templating G. Individual data points and error bars represent mean ± s.d. An uncropped gel image for d and data behind the graph in e are available as source data.
Source data
Full size image
Elongation assays showed that the M residue at the +1 position of the template strand directed incorporation of either G or A into nascent RNA, but not C or U (Fig. 3d,e). This can be explained by the formation of M-GTP or M-ATP base pairs in the RdRp active center. Consistent with this, thermal melting experiments with RNA duplexes containing M-G or M-A base pairs located at terminal or internal positions showed similar RNA duplex stabilities that were slightly lower than for duplexes containing a C-G base pair (Extended Data Fig. 2 and Supplementary Table 2). Thus, when the RdRp uses RNA containing NHC monophosphate as a template, either the correct or the incorrect nucleotide is incorporated into the RNA product, and thus mutagenesis will occur.
Structural basis of NHC-induced RNA mutagenesis
The above data indicate that the key aspect of the mutagenesis mechanism is the formation of stable M-G and M-A base pairs in the RdRp active center. To investigate this, we solved two structures of RdRp–RNA complexes that correspond to mutagenesis products after M-templated incorporation of either G or A (Methods). We formed RdRp–RNA complexes containing M in the template strand and either G or A at the 3′ end of the product strand. This was predicted to result in the formation of nascent M-G or M-A base pairs in position −1, which is occupied after successful M-templated nucleotide incorporation and RdRp translocation. We prepared RNA duplex scaffolds with M-containing oligonucleotides (Extended Data Fig. 3), formed RdRp–RNA scaffold complexes, and subjected these to cryo-EM analysis as described in ref. 15.
We indeed obtained RdRp–RNA structures that contained either an M-A or an M-G base pair at position −1 (Fig. 4 and Table 1). The structures showed an overall resolution of 3.3 Å and 3.2 Å, respectively, with the active center region resolved at ~2.9 Å in both cases (Extended Data Fig. 4). As expected from the scaffold design, the structures showed the post-translocation state with a free NTP-binding site at position +1 (Fig. 4b,c). Comparison of the two structures with each other and with our original RdRp–RNA structure15 and with remdesivir-containing RdRp–RNA structures23 did not reveal major differences, neither in the protein subunits nor in the nucleic acids, except that the protruding, second turn of RNA and the sliding poles of the nsp8 subunits were poorly ordered and not retained in the final model.
Fig. 4: Structures of RdRp–RNA product complexes after NHC-induced mutagenesis.
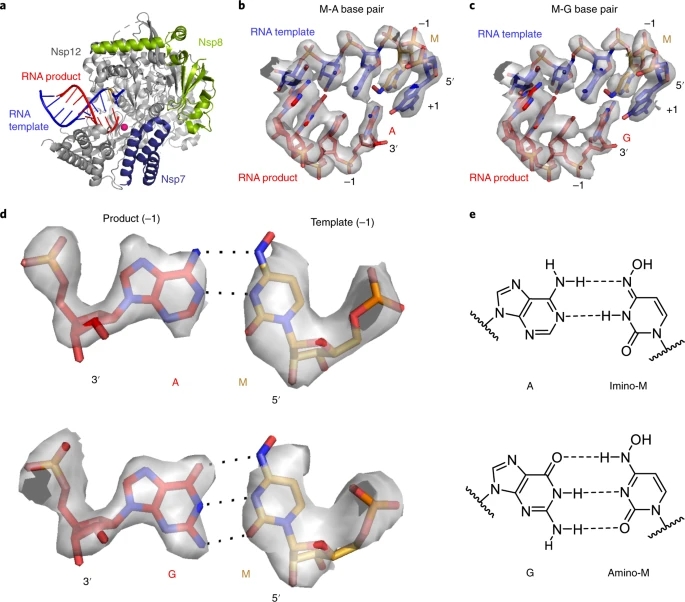
figure 4
a, Overview of RdRp–RNA structure with an M residue (orange) at position −1 in the RNA template strand. RdRp subunits nsp7, nsp8 and nsp12 are in dark blue, green and gray, respectively. The RNA template and product are in blue and red, respectively. The active site is indicated by a magenta sphere. Depicted is the structure containing the M-A base pair. b, RNA duplex containing the M-A base pair in the RdRp active center. The +1 position (templating nucleotide, NTP substrate site) and the −1 position (post-translocation position of the nascent base pair) are indicated. c, RNA duplex containing the M-G base pair in the RdRp active center. d, Cryo-EM density for the nascent M-A (top) and M-G (bottom) base pairs in position −1, viewed along the RNA duplex axis in the direction of RNA translocation. e, M-A (top) and M-G (bottom) base pairing relies on different tautomeric forms of NHC45, as predicted46.
Full size image
Table 1 Cryo-EM data collection, refinement and validation statistics
The cryo-EM densities at the −1 position of the structures could readily be interpreted by modeling M-A or M-G base pairs (Fig. 4b,c). The densities were so detailed that we could clearly distinguish G and A bases (Fig. 4d). The densities were also consistent with the proposed base pairing46 that is enabled by different tautomeric forms of NHC45 (Fig. 4e). However, the observed hydrogen-bonding geometries were not optimal, possibly explaining our biochemical observations that suggest that M can mimic C well, and mimic U to some extent, but neither mimicry is perfect (Figs. 1 and 2). These results represent the first direct visualization of NHC in a polymerase enzyme and show that stable M-G and M-A base pairs can be formed and accommodated in the RdRp active center, readily explaining our biochemical results.
Discussion
Our systematic biochemical analysis suggests a two-step model for the mechanism of molnupiravir-induced coronavirus RNA mutagenesis (Fig. 5). When the molnupiravir prodrug enters the cell, it is converted to NHC triphosphate (MTP), which can be used by the RdRp of SARS-CoV-2 as a substrate instead of CTP or UTP. Therefore, in a first step, the RdRp is predicted to frequently incorporate M instead of C or U when it uses the positive-strand genomic RNA (+gRNA) as a template to synthesize negative-strand genomic (−gRNA) and subgenomic RNA (−sgRNA). In a second step, the resulting M-containing RNA can be used as a template for the synthesis of +gRNA or positive-strand subgenomic mRNA (+sgmRNA). The presence of M in the −gRNA then leads to mutations in the positive-strand RNA products, which do not s
R&D Center: API Production Base of Technical Economic
Development Area, Shanghe County, Jinan, China
Sales Center: No. 322 of Tongke building,
high-tech Zone,Jinan, China
HR TEL: 86-531-58565868
TEL: 86-15653103076
86-15020285806(global market)
86-18369000022(domestic market)
E-MAIL: xinhuang@sddhpharm.com